Abstract
Objective
To determine incidence of venous thromboembolism (VTE) and to describe risk factors and demographics at a community hospital setting.
Methods
Prospective study design, recruited 33 patients at Orange Park Medical Center (OPMC). Study was conducted for a period of two months from Feb 2017 to March 2017.
All patients aged 18 years and above with an objectively verified diagnosis of DVT, PE or both were included in the study.
Baseline demographic data were retrieved from medical records and descriptive analysis was performed with R.
Results:
During the study period, the incidence rate of VTE diagnoses was 5.9 per 1000 admits [95% confidence interval (CI): 4- 8.2]. Mean age of the patients with VTE event was 51 years, majority were females (51.5%) and Caucasians (69.6%). The average length of stay in the hospital was 3.42 days.
Incidence rate for DVT was 1.3 per 1000 admits [95% CI: 1.3- 4.1] and that for PE was 3.7 per 1000 admits [95% CI: 2.3- 5.7]. DVT's were more common among males (57.1%) compared to females (42.8%), whereas PE's were more common in females (66.66%) compared to males (33.33%).
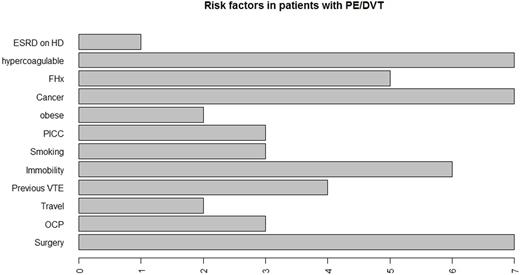
Majority of VTE were provoked episodes, accounting for nearly 64% of all causes. Remaining 36% of VTE events were unprovoked due to hypercoagulable states (21%) and a family history of VTE (15%). Among the provoked events, most common causes were malignancy (21%) and recent surgeries (21%) (Figure 1).
Among the patients whose risk factor for VTE was recent surgery, majority of them had orthopedic surgeries (57.1%) and received low molecular weight heparins (LMWHs) as prophylaxis for an average duration of two weeks post- surgery.
Our results also showed that, 51.5% of patients diagnosed with VTE had at least 1 risk factor for VTE and 48.4% had 2 or more risk factors.
Conclusion
This study provides an estimate of incidence of VTE events in adults at a community hospital setting. A sample size of 5590 hospitalized adult patients were analyzed, of which 33 patients were diagnosed with VTE. There have been very few studies reporting the simultaneous effects of multilevel factors such as demographic and preexisting comorbid conditions on the incidence of VTE. When compared to a previous study on VTE in hospitalized patients by Tsai J et al 1, we found a slightly increased incidence rate of 5.9 VTE's per 1000 admits. The difference in the incidence rate could be due to differences in clinical practice of VTE prophylaxis among clinicians, patient's attitudes towards compliance and adherence to therapies. In regards to the risk factors, our study results were consistent with existing evidence that there is a greater risk for VTE among those patients with malignancies and those who have had recent surgeries 2.
Patients undergoing major orthopedic surgery are at particularly high risk for VTE. The current pharmacological recommendations for prophylaxis of VTE are LMWHs, fondaparinux and vitamin K antagonists. The results of several studies support extended prophylaxis for 4 weeks in patients undergoing high risk orthopedic surgeries and surgery for cancer. The optimal duration of VTE prophylaxis in other types of surgery needs further evaluation 3.
In summary, the results from this study have several important clinical and public health implications. First, physicians need to be informed of various risk factors for VTE, and to identify those who will benefit from VTE prophylaxis. Second, to increase the awareness of VTE among patients and thereby contribute to improved adherence to prescribed therapies.
References
1. Tsai, J., Grant, A., Beckman, M., Grosse, S., Yusuf, H. and Richardson, L. (2015). Determinants of Venous Thromboembolism among Hospitalizations of US Adults: A Multilevel Analysis. PLOS ONE, 10(4), p.e0123842
2. Abdel-Razeq, H. (2010). Venous thromboembolism prophylaxis for hospitalized medical patients, current status and strategies to improve. Annals of Thoracic Medicine, 5(4), p.195
3. Agnelli, G. (2004). Prevention of Venous Thromboembolism in Surgical Patients. Circulation, 110(24_suppl_1), pp.IV-4-IV-12.
Moezi: BMS: Speakers Bureau; Novartis: Speakers Bureau; Pfizer: Speakers Bureau; Takeda: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal